
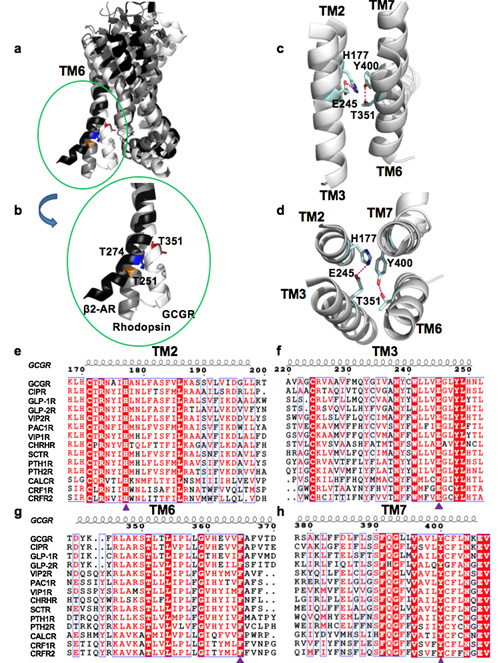
B型GPCR的TM2、TM3、TM6和TM7的保守氨基酸通过形成极性口袋来稳定受体的未激活构象。
论文链接
论文摘要
The glucagon receptor (GCGR) belongs to the secretin-like (class B) family of G protein-coupled receptors (GPCRs) and is activated by the peptide hormone, glucagon. The structures of an activated class B GPCR have remained unsolved, preventing a mechanistic understanding of how these receptors are activated. Using a combination of structural modeling and mutagenesis studies, we present here two modes of ligand-independent activation of GCGR. First, we identified a GCGR-specific hydrophobic lock comprising M338 and F345 within the IC3 loop and transmembrane helix 6 (TM6) and found that this lock stabilizes the TM6 helix in the inactive conformation. Disruption of this hydrophobic lock led to constitutive G protein and arrestin signaling. Second, we discovered a polar core comprising conserved residues in TM2, TM3, TM6, and TM7, and mutations that disrupt this polar core led to constitutive GCGR activity. On the basis of these results, we propose a mechanistic model of GCGR activation, in which TM6 is held in an inactive conformation by the conserved polar core and the hydrophobic lock. Mutations that disrupt these inhibitory elements allow TM6 to swing outwards to adopt an active TM6 conformation similar to that of the canonical β2 adrenergic receptor complexed with G protein and to that of rhodopsin complexed with arrestin. Importantly, mutations in the corresponding polar core of several other members of class B GPCRs, including PTH1R, PAC1R, VIP1R, and CRFR1, also induce constitutive G protein signaling, suggesting that the rearrangement of the polar core is a conserved mechanism for class B GPCR activation.